Hey there! Let’s dive into the world of molecular geometries and explore the difference between trigonal planar and trigonal pyramidal shapes. Don’t worry, we’ll keep it light and easy to understand, just like a casual conversation.
Trigonal Planar
Trigonal planar geometry is all about simplicity and symmetry. Imagine a central atom sitting in the middle, with three atoms or groups of atoms arranged around it in a flat, triangular shape. These peripheral atoms are positioned at the corners of an equilateral triangle, and the bond angles between them are all 120 degrees. This flat arrangement is why it’s called “planar.”
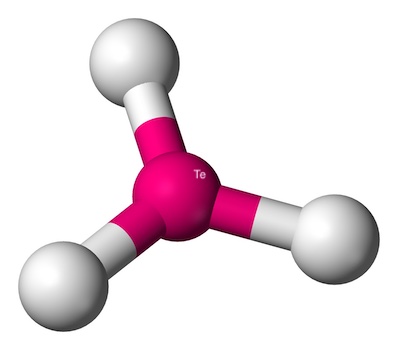
Here are some key points about trigonal planar geometry:
- No Lone Pairs: The central atom doesn’t have any lone pairs of electrons. This means there’s no extra electron pair to push the bonded atoms out of the plane.
- Bond Angles: The angles between the bonds are a perfect 120 degrees.
- Examples: Common examples include boron trifluoride (BF3) and formaldehyde (CH2O). These molecules have a beautiful, flat triangular shape.
Trigonal Pyramidal
Now, let’s talk about trigonal pyramidal geometry. This shape is a bit more dynamic and three-dimensional. Here, the central atom is surrounded by three atoms or groups of atoms, just like in trigonal planar, but there’s a twist: the central atom also has a lone pair of electrons. This lone pair pushes the bonded atoms down, creating a pyramid-like shape.
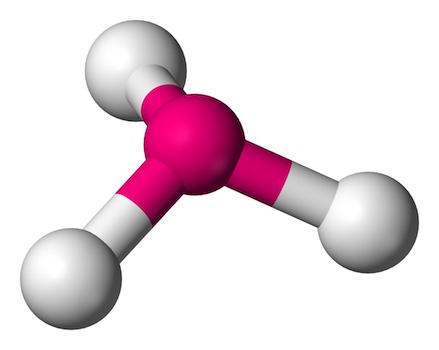
Key features of trigonal pyramidal geometry include:
- One Lone Pair: The central atom has one lone pair of electrons, which influences the shape by pushing the bonded atoms into a pyramid.
- Bond Angles: The angles between the bonds are slightly less than 109.5 degrees due to the repulsion from the lone pair, typically around 107 degrees.
- Examples: Ammonia (NH3) and phosphine (PH3) are classic examples. The lone pair on the nitrogen or phosphorus atom creates a distinct pyramidal shape.
Comparing the Two
Let’s put these two geometries side by side and highlight their differences:
| Feature | Trigonal Planar | Trigonal Pyramidal |
|---|---|---|
| Lone Pairs | None | One |
| Bond Angles | 120 degrees | Around 107 degrees |
| Planarity | Atoms lie in a single plane | Atoms do not lie in a single plane |
| Shape | Flat, triangular | Three-dimensional, pyramid |
| Examples | BF3, CH2O | NH3, PH3 |
| Repulsion | Bond-bond repulsion | Bond-bond and bond-lone pair repulsion |
| Hybridization | sp2 | sp3 |
Why It Matters
Understanding these geometries is important for predicting the properties and behaviors of molecules. For instance, the presence of a lone pair in trigonal pyramidal molecules often makes them more polar and reactive compared to their trigonal planar counterparts. This can influence how these molecules interact with others, their stability, and even their physical properties like boiling and melting points.
Visualizing the Shapes
To help visualize, think of a trigonal planar molecule as a flat triangle, like a slice of pizza with equal sides. Now, imagine trigonal pyramidal as a pyramid or a tripod where the central atom is at the top, and the three bonded atoms form the base.
Wrapping Up
In summary, the main difference between trigonal planar and trigonal pyramidal geometries lies in the presence of a lone pair of electrons on the central atom in trigonal pyramidal molecules. This lone pair creates a three-dimensional shape with slightly smaller bond angles, while trigonal planar molecules maintain a flat, triangular shape with equal bond angles.
Understanding these differences can help you grasp why certain molecules behave the way they do, making your chemistry journey a bit more interesting and enjoyable!
Feel free to ask if you have any more questions or need further clarification on this topic!





